Ammonia:
Meanwhile,
the process in another plant:
Urea:
Stoichiometrically, a mole of nitrogen react with three moles of hydrogen gas produces two moles of ammonia through exothermic reaction. However, this reaction found to be unfavourable in certain condition through physical factors such as activation energy, pressure, temperature and catalyst. Therefore, Le Chatelier’s Principle was applied to increase production of ammonia as reaction is irreversible in nature. First, reactants flow over iron catalyst supported by potassium hydroxide promoter to lower activation energy. Increase in pressure typically 200 atm used allow reaction to shift towards low moles producing more ammonia and low temperature approximately 400-450℃ favour ammonia production. Haber process primarily focuses on ammonia production, but today, 80% of ammonia manufactured as feedstock for urea development which is a more stable nitrate to produce fertilizer (Alkusayer et al., n.d.). The first commercial ammonia plant based on Haber-Bosch process built by BASF at Oppau, Germany. The plant started on stream on Sept. 9 1913 with production capacity of 30 m.t./day (Introduction to Ammonia Production | AIChE, n.d.). Figure 1 below shows a flow diagram of commercial ammonia production by BASF:
As increasing demand of synthetic
ammonia for fertilizer production and Haber-Bosch method usage over centuries,
this resulted in countless development and modifications. Development of
Haber-Bosch process creating advancement of large-scale production, high-pressure
technology, continuous-flow and according to Alkusayer et al., production of
ammonia has reached 159 tonnes annually, approximately 83% for manufacture of
fertilizers needed for agriculture. As a result, advanced in technologies alter
the process or equipment while maintaining its general process. There are six
general steps for ammonia production: catalytic steam reforming, natural gas
desulfurization, carbon dioxide removal, carbon monoxide shift, ammonia
synthesis and methanation. After multiple
passes of Harber process, 97% of reactants were converted overall. While
nitrogen reacted from air, a bulk of hydrogen gas and carbon monoxide were
produced through catalytic steam reforming of natural gas where steam reacted
with natural gas (methane) at high temperature ranging 700-1100℃. Production ammonia is approximately 98% generated. Figure 2 shows
flow diagram of typical ammonia production today.
Figure
2. Flow diagram of ammonia production process
CATALYST USED IN HABER PROCESS
Figure 3: Catalytic Process using Iron Catalyst
Though Haber-Bosch process has been used extensively for production of nitrogenous fertilizers to satisfy agricultural demands, the consequences of growth of crops in large quantity and chemical emissions has inspired engineers to seek and design alternative methods for fertilizer production. Therefore, the substitute to Haber-Bosch process of ammonia through heterogenous catalyst development is a Never-Ending-Story (Marakatti & Gaigneaux, 2020).
References:
1. Alkusayer,
K. T., Ollerhead, A., & Kmiotek, S. J. (n.d.). Ammonia Synthesis for
Fertilizer Production Written by Advised by. Retrieved January 4, 2021,
from http://www.wpi.edu/academics/ugradstudies/project-learning.html
2. Introduction
to Ammonia Production | AIChE. (n.d.). Retrieved January 5, 2021, from
https://www.aiche.org/resources/publications/cep/2016/september/introduction-ammonia-production
3. Marakatti,
V. S., & Gaigneaux, E. M. (2020). Recent Advances in Heterogeneous
Catalysis for Ammonia Synthesis. In ChemCatChem (Vol. 12, Issue 23, pp.
5838–5857). Wiley Blackwell. https://doi.org/10.1002/cctc.202001141

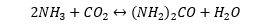



No comments:
Post a Comment